Adsorption
What is it?
Adsorption is a heterogeneous reaction in which gas molecules are retained on a solid or liquid surface (adsorbent also referred to as a molecular sieve) that prefers specific compounds to others and thus removes them from effluent streams (Figure 1). When the surface has adsorbed as much as it can, the adsorbed content is desorbed as part of the regeneration of the adsorbent. When desorbed, the contaminants are usually at a higher concentration and can either be recovered or disposed of.
Typical adsorbents include (EIPPCB; 2016):
Typical adsorbents include (EIPPCB; 2016):
- granular activated carbon (GAC), the most common adsorbent with a wide efficiency range and not restricted to polar or non-polar compounds; GAC can be impregnated, e.g. with oxidants such as potassium permanganate;
- zeolites, properties depending on their manufacturing, working either as mere molecular sieves, selective ion exchangers or hydrophobic VOC adsorbers;
- macroporous polymer particles, which are used as granules or beads, without being highly selective with respect to VOCs;
- silica gel;
- sodium-aluminium silicates.
- fixed-bed adsorption;
- fluidised-bed adsorption;
- continuous moving-bed adsorption;
- pressure swing adsorption (PSA).
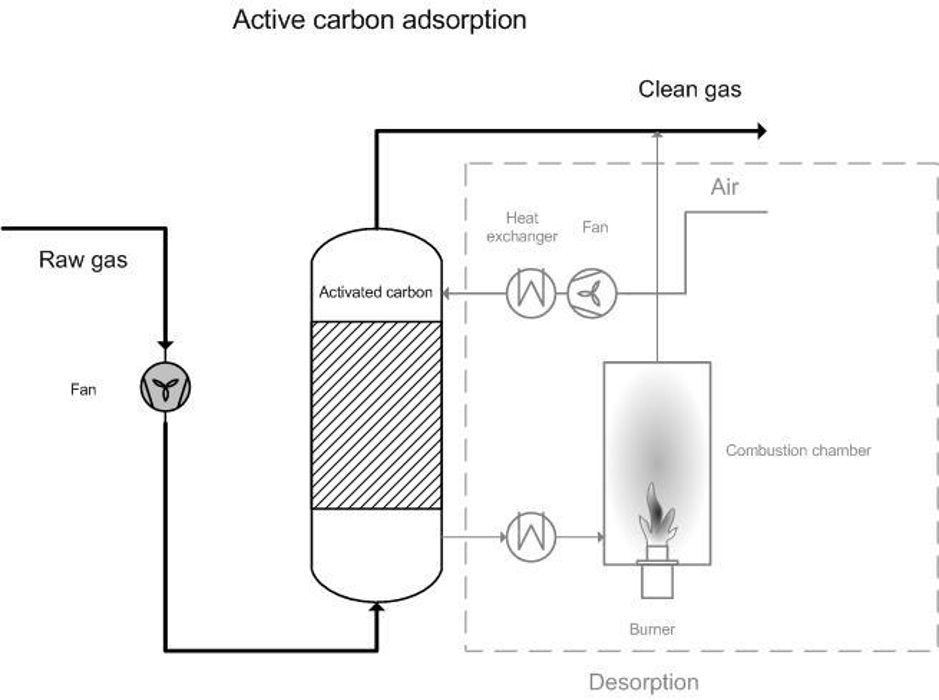
Figure 1. Diagram of active carbon adsorption system
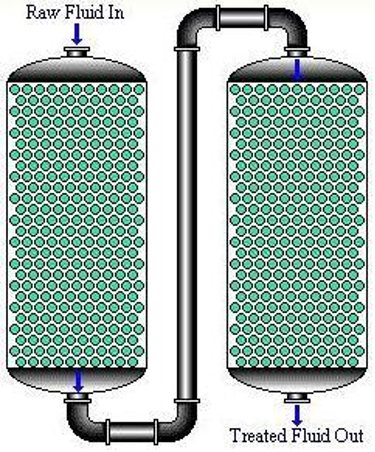
Figure 2. Examples of fixed bed columns: in series (left) and in parallel (right)
Design, maintenance and efficiency
Since all adsorption processes are exothermic, they cause a temperature rise, which is not desirable for the adsorption of organic compounds. Carbon or metals on GAC as well as zeolites can catalyse the oxidation of some compounds when the adsorbent is hot, resulting in bed fires that also consume part or all of the GAC, but not the zeolite. This is a hazard when adsorbing certain hydrocarbons (such as ketones or comparable active compounds) at ambient temperatures that are close to those that cause the organic compound to oxidise. Such a GAC bed fire can either alter the pore size of the remainder of the bed, or oxidise the bed to ash, which is a serious incident that may burn down the whole facility. These fires may be suppressed by humidification of the air and by intentional cooling of the GAC.
Temperature monitoring of the gas outlet of the GAC adsorber is required to prevent fire risk. Another important measurement is the pressure drop across the adsorbent bed. Across the bed, the pressure should remain roughly constant. There should be an alarm for high pressure.
Temperature monitoring of the gas outlet of the GAC adsorber is required to prevent fire risk. Another important measurement is the pressure drop across the adsorbent bed. Across the bed, the pressure should remain roughly constant. There should be an alarm for high pressure.
Applicability
Table 1 shows application limits and restrictions associated with adsorption (adapted from EIPPCB, 2016, Table 3.166).
Table 1. Application limits and restrictions associated with adsorption.
NI = no information available
Table 1. Application limits and restrictions associated with adsorption.
| Issue | GAC | Zeolites |
|---|---|---|
| Gas flow (Nm3/h) | 100-100000 | <100000 |
| Temperature (°C) | 15-80 (ideally about 20) | <250 |
| Pressure (MPa) | 0.1-2 | Atmospheric |
| Pressure drop (mbar) | 10 to 50 | NI |
| Odour concentration (ouE/m3) | 5000-100000 | NI |
| Dust content (mg/Nm3) | Low concentration to prevent obstruction | Low concentration to prevent obstruction |
| Relative humidity of the waste gas | max 70% | NI |
