Ionisation
What is it?
In ionisation (also referred to as direct cold plasma technique), the air or the incoming gas flow is led through a reaction chamber where it is submitted to a very strong electrical field (20–30 kV) generated by electrodes, causing ions, free electrons, radicals and other highly reactive particles to be formed. However, no notable rise in temperature takes place.
The highly reactive compounds cause the decomposition and (partial) oxidation of the pollutants present in the incoming gas. The most active particles in this process are the N, O and OH radicals. They are formed of nitrogen (N2), oxygen (O2) and water (H2O). With direct treatment, the removal of organic chemicals is possible. In the case of injection of an ionised air stream, a modification of the odour molecules occurs and, to a lesser extent, a removal of the organic load.
The highly reactive compounds cause the decomposition and (partial) oxidation of the pollutants present in the incoming gas. The most active particles in this process are the N, O and OH radicals. They are formed of nitrogen (N2), oxygen (O2) and water (H2O). With direct treatment, the removal of organic chemicals is possible. In the case of injection of an ionised air stream, a modification of the odour molecules occurs and, to a lesser extent, a removal of the organic load.
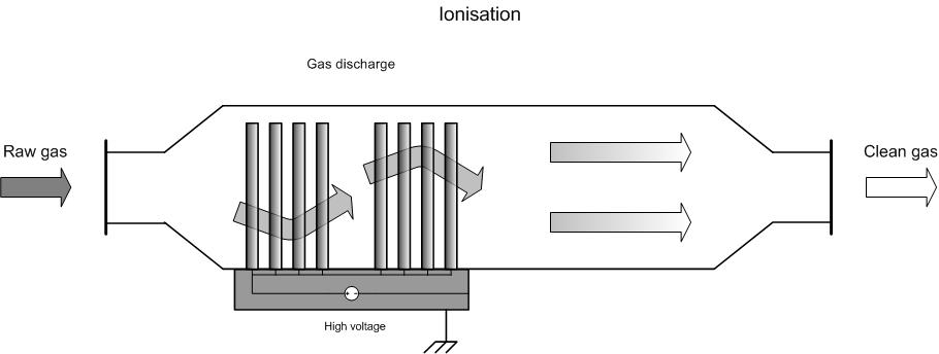
Figure 1. Diagram of an ionisation system
The decomposition of VOCs can generate emissions of CO2, H2O2, CO, NOX, etc. which can be treated using a catalyst system.
Ozone created in the electrical field is a side product. If it is not completely reacted it leads to ozone emissions. Ozone has a distinctive smell and can be harmful in high concentrations. Under normal atmospheric conditions, ozone is quickly transformed into oxygen. When placing a catalyst in series after the ioniser, the ozone is completely removed. In industrial applications, the ozone emission stays below one ppm.
Waste water is emitted as a small quantity of drainage water.
Ozone created in the electrical field is a side product. If it is not completely reacted it leads to ozone emissions. Ozone has a distinctive smell and can be harmful in high concentrations. Under normal atmospheric conditions, ozone is quickly transformed into oxygen. When placing a catalyst in series after the ioniser, the ozone is completely removed. In industrial applications, the ozone emission stays below one ppm.
Waste water is emitted as a small quantity of drainage water.
Design, maintenance and efficiency
The maintenance is minimal. If the unit is used to abate odour, a 'wash-down' once a week may be needed and an internal inspection once a month would be recommended.
Voltage is the main parameter that is to be monitored.
Voltage is the main parameter that is to be monitored.
Applicability
Ionisation is normally used to treat waste gases with low concentrations of VOCs and in cases where thermal/catalytic oxidation is not effective.
The first prototype applications of plasma oxidation for air purification at an industrial scale date back to the late '80s. The technology has been fully commercialised since the mid-‘90s. In the meantime, dozens of such installations are being used for odour control, including in the following sectors:
Table 1 shows application limits and restrictions associated with ionisation (adapted from EIPPCB, 2016, Table 3.214).
Table 1. Application limits and restrictions associated with ionisation.
- Water purification (RWZI, food, chemicals and leather industry);
- Sludge composting;
- Tobacco industry;
- Foodstuffs industry;
- Fish-feed industry;
- Animal-feed industry;
- Slaughterhouses;
- Grain and soya processing;
- Potato processing (crisps production).
Table 1 shows application limits and restrictions associated with ionisation (adapted from EIPPCB, 2016, Table 3.214).
Table 1. Application limits and restrictions associated with ionisation.
| Issue | Limits/ Restrictions |
|---|---|
| Gas flow (Nm3/h) | 20-200000 |
| Temperature (°C) | 20-80; Higher temperatures are possible (up to 120) with plasma oxidation |
| Pressure (MPa) | Atmospheric |
| Pressure drop (mbar) | Some |
| Relative humidity (%) | Not too high because of risks of condensation and short- circuiting. A heightened humidity improves the performance in a side stream set-up |
| Dust concentration | If applied directly into the gas stream, this should include relatively low amounts of dust. The ioniser will then act as an electrostatic precipitator |
| Energy | Ionisation is primarily suited for gas streams with low concentrations of VOCs because of the low energy consumption compared to thermal oxidisers |
