Photo/ UV Oxidation
What is it?
The incoming waste gas stream is led through a reaction chamber and radiated with UV waves (100–280 nm). This radiation causes the decomposition of the undesired compounds.
This decomposition takes place in two ways:
This decomposition takes place in two ways:
- Photolysis: compounds such as VOCs, NH3 H22 and amines are directly broken down by the radiation;
- Oxidation by reactive oxygen radicals: the presence of highly reactive oxygen radicals oxidises compounds that are not broken down by direct photolysis and reaction products from the photolysis.
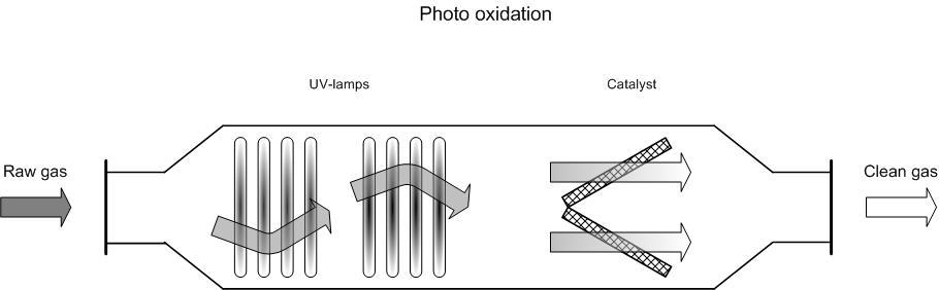
Figure 1. Diagram of a photo oxidation system
Design, maintenance and efficiency
Some suppliers install a catalyst, an adsorption system (activated carbon), or a second set of lamps with a different wavelength after the first photo oxidation phase to reach the highest possible removal rate. This extra phase also serves to break down the remaining ozone into oxygen.
Besides spent UV lamps (expected lifespan of about 8000 hours), no waste is created. The energy consumption is in the range of 0.3–1.5 kWh/1000 Nm3.
Besides spent UV lamps (expected lifespan of about 8000 hours), no waste is created. The energy consumption is in the range of 0.3–1.5 kWh/1000 Nm3.
Applicability
Photo oxidation is particularly suited to discontinuous processes with lower solvent concentrations (maximum 500 mg/Nm3). The process reaches its steady-state removal yield almost immediately and has no extra start-up costs or disadvantages compared to continuous operation.
The first use of photo oxidation in air purification on an industrial scale dates back to the late ‘90s. Applications can be found in the following sectors:
Table 1 shows application limits and restrictions associated with photo/ UV oxidation (adapted from EIPPCB, 2016, Table 3.218).
Table 1. Application limits and restrictions associated with photo/ UV oxidation.
NI = no information available
The first use of photo oxidation in air purification on an industrial scale dates back to the late ‘90s. Applications can be found in the following sectors:
- Coating installations;
- Wastewater treatment;
- Waste sorting/ processing installations;
- Fermentation processes, breweries;
- Foodstuffs industry (meat, fish);
- Kitchens.
Table 1 shows application limits and restrictions associated with photo/ UV oxidation (adapted from EIPPCB, 2016, Table 3.218).
Table 1. Application limits and restrictions associated with photo/ UV oxidation.
| Issue | Limits/ Restrictions |
|---|---|
| Gas flow (Nm3/h) | 2000-58000 (in theory not very critical) |
| Temperature (°C) | < 60 |
| Pressure (MPa) | Atmospheric |
| Pressure drop (mbar) | NI |
| Relative humidity (%) | Dust removal should preferably be carried out |
| Dust concentration | Dust removal should preferably be carried out |
| Energy | Ionisation is primarily suited for gas streams with low concentrations of VOCs because of the low energy consumption compared to thermal oxidisers |
